Citation:
Date Published:
NOV 12Abstract:
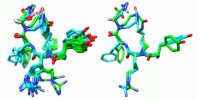
Painkillers are commonly used medications. Native peptide painkillers suffer from various pharmacological disadvantages, while small molecule painkillers like morphine are highly addictive. We present a general approach aimed to use backbone-cyclization to develop a peptidomimetic painkiller. Backbone-cyclization was applied to transform the linear peptide Tyr-Arg-Phe-Sar (TAPS) into an active backbone-cyclic peptide with improved drug properties. We designed and synthesized a focused backbone-cyclic TAPS library with conformational diversity, in which the members of the library have the generic name TAPS c(n-m) where n and m represent the lengths of the alkyl chains on the nitrogens of Gly and Arg, respectively. We used a combined screening approach to evaluate the pharmacological properties and the potency of the TAPS c(n-m) library. We focused on an in vivo active compound, TAPS c(2-6), which is metabolically stable and has the potential to become a peripheral painkiller being a full mu opioid receptor functional agonist. To prepare a large quantity of TAPS c(2-6), we optimized the conditions of the on-resin reductive alkylation step to increase the efficiency of its SPPS. NMR was used to determine the solution conformation of the peptide lead TAPS c(2-6).
Notes: